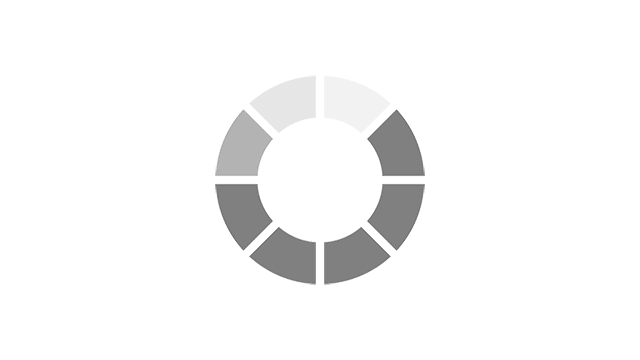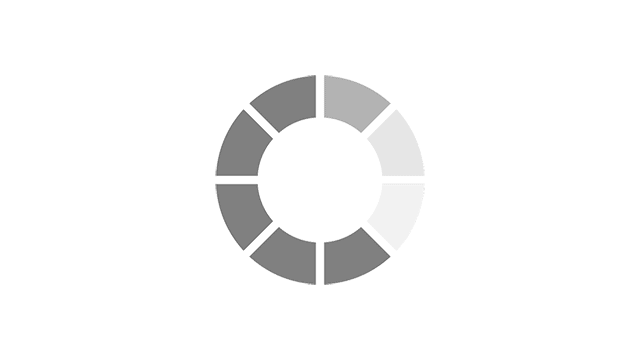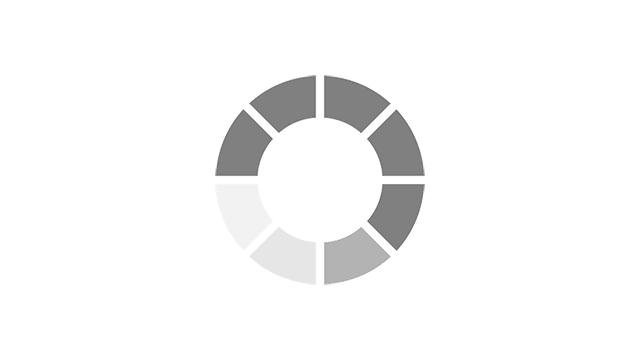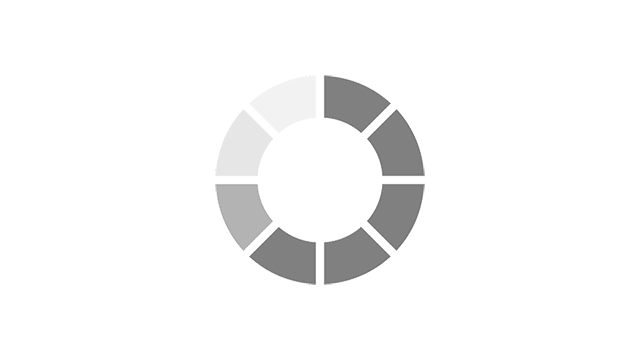
Menopause symptoms experienced in the past week using MENQOL* EU5 countries, n= 20351.

Adapted from Nappi RE, et al. Menopause 2021;28(8):875–882.
*MENQOL, Menopause-Specific QOL (questionnaire to assess QOL and impact of menopause symptoms)2

VMS: Vasomotor Symptoms, also known as night sweats and hot flushes.
Reduction from baseline was a mean of 2.9 for night-time VMS and a mean of 4.1 for daytime VMS. Data are from a prespecified pooled analysis of the Phase 3 studies SKYLIGHT 1 and 2.
Sleep problems during menopause can lead to substantial impact on quality of life and ability to function the next day7. This is often measured using MENQOL, the Menopause-Specific QOL questionnaire to assess QOL and impact of menopause symptoms2.
With Veoza, there was a greater improvement in QoL (MENQOL) compared with placebo4,5,6
Mean change in MENQOL Total Scorea from Baseline to Week 12 in SKYLIGHT 1&2 Pooled Analysis

aComprises all 4 domains and 29 items. Negative change indicates improvement from baseline.
In 1 week, patients taking VEOZA experienced a reduction in the number of VMS episodes, which was sustained through 52 weeks5,8

Graph produced using the FAS.5,9
The recommended dose of VEOZA is 1 tablet taken orally with or without food3

It's time to discuss VEOZA with your patients today.
Fast relief of hot flushes & night sweats. 1 tablet. 1 dose. 1 per day.
MAT-IE-VEO-2025-00044 June 25